Epigenetics helps explain how your experiences and your environment can affect your health and the health of future generations, beyond just your genes. It’s how you can change which genes you turn on or off – without changing the actual sequence of DNA code. Instead of changing the actual DNA code, epigenetics modifies the DNA molecules via the chemistry surrounding the DNA. This post breaks down the mechanisms that create epigenetic changes in the body into 5 steps, specifically using exercise as an example of an epigenetic influence.
STEP ONE: Some behavior triggers a molecular implication
Practically everything (possibly actually everything) has a molecular impact. Well-examined behaviors that trigger epigenetic implications include nutrition, mental-emotional stress, working habits, smoking, lifestyle, environmental toxins, sleep habits, obesity, and physical exercise.

Several molecular implications that exercise may cause, include Calcium Flux, ATP utilization, and Oxidative Stress. These three will be explained below.
Calcium Flux
In order to understand calcium flux, it is helpful to understand the chemistry of muscular contraction. In muscles that are not actively contracting, regulatory proteins “hang out” preventing the interaction of the actin and myosin filaments. In order to contract your muscles, a motor neuron sends an electrical signal to the cell membrane, activating the voltage-gated calcium channel, allowing calcium to flow into the muscle cell. Calcium must diffuse into the cytoplasm in this manner in order to interact with the regulatory proteins, which causes the actin and myosin filaments to slide into each other, thus triggering the contraction of the muscle cell.,
This calcium flow, or flux, is an example of a potential mechanism that may trigger epigenetic changes in the cellular DNA environment.
ATP Utilization (ATP:AMP Ratio)
ATP (tri-phosphate) is an “energy-carrying molecule found in the cells of all living things.” When you exercise, you need energy, so ATP consumption is increased by breaking off the phosphate tail, which resultantly alters the ratios of ADP (di-phosphate) and AMP (mono-phosphate).
Ultimately, increasing ATP consumption causes a rise in AMP. “A high ratio of AMP:ATP is a signal that the energy status of the cell is compromised.”
This AMP:ATP ratio is another example of a potential mechanism that may trigger epigenetic changes in the cellular DNA environment.
Oxidative Stress
Oxidative stress is an imbalance between the accumulation of a kind of free radical called reactive oxygen species (ROS), and the ability of the system to detoxify them. Exercise increases oxidative stress in the short term yet decreases it over the long term. These shifts in oxidative stress are another example of a potential mechanism that may trigger epigenetic changes in the cellular DNA environment.
STEP TWO: Natural gene expression occurs
Genes are constantly expressing. The “central dogma” where DNA creates RNA which creates Proteins, is constantly occurring.

In other words, DNA is constantly sending RNA messengers around the cells (thousands per second), with instructions for creating various proteins to perform all of the important genetic and cellular processes that our genes and cells are responsible for! Two specific examples of gene expression include:
- Pancreatic β-cells expressing insulin hormone in response to rising levels of glucose
- Liver cells expressing alcohol dehydrogenase enzyme to detoxify alcohol
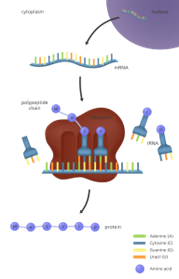
Image: Genome Research Limited
STEP THREE: Environment for epigenetic change
Some natural end-products of gene expression have epigenetic influence. Enzymes & Transcriptional Regulators/Factors, floating around in the cell, combine to trigger what we refer to as epigenetic changes in the DNA.
These enzymes, regulators, and factors (sometimes subcategorized as activators or repressors), are categories of proteins that are translated during usual gene expression. Examples include:
- Activator PGC-1α – A protein that regulates the genes involved in energy metabolism.
- “Exercise strongly induces PGC-1α expression in muscle.”
- Exercise increases the muscle cell’s metabolic demand. The production of PGC-1α is thought to help meet the metabolic demand that increases as a result of physical activity. “PGC-1α fulfills this in a number of ways, such as increasing mitochondrial biogenesis, thus increasing the capacity for generation of ATP, or increasing angiogenesis [formation of new blood vessels], thus providing more nutrients to the muscle.”
- Repressors NCoR & SMRT – Proteins that regulate estrogen and androgen receptors. Multiple studies have looked at the effects of NCoR & SMRT on various body functions.
- SMRT – regulates a significant but still not understood pathway that is critical to body weight regulation.
- NCoR levels are decreased as a result of exercise which may lead to an increase in genes involved with energy metabolism and fat burning.
- Rat studies have shown that a single session of exercise changes the activity of HDAC proteins, which require NCoR and SMRT in order to exert their epigenetic influence.
- Enzymes of the DNMT family – Enzymes that establish methylation of CpG islands by blocking accessibility to transcriptional activators.
- “A single exercise session can decrease DNMT1 and DNMT3b levels in the hippocampus of young adult rats,” which affects DNA methylation in a manner that “delay[s] the processes of aging and neurodegeneration.”
- Enzyme AMPK – Enzymes that play a role in cellular energy homeostasis.
- Physical exercise stimulates AMPK. “AMPK activation through physical exercise improves mitochondrial biogenesis through the regulation of peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), which promotes the expression of mitochondrial genes.”
- Enzyme mTOR – A protein kinase enzyme that regulates cell growth, cell proliferation, cell motility, cell survival, anabolic metabolism, and more.
- “Resistance exercise can induce an increase in protein synthesis through an mTOR-dependent process,” thus “the rapamycin-sensitive components of mTOR signaling have been widely implicated in the pathway through which resistance exercise induces skeletal muscle hypertrophy.”
- Enzyme SIRT 1 – Enzymes that are considered very important for stress and longevity, are “considered a promising target for treating human diseases.”
- “An acute load of exercise activates SIRT1.
- Enzyme PDK – regulates activity of other enzymes, to help regulate glycolysis and ATP generation.
- “PDK activity approximately doubled after 8 weeks of aerobic training.”
So, “physical exercise alters gene expression as cells adapt to the metabolic change during a bout of exercise,” but if exercise is not regular, these epigenetic adaptations will regress.
STEP FOUR: Genes turn ON or OFF
In this step, the end products that alter the cellular/DNA environment are now set to alter the next round of gene expression. Two of the most described epigenetic changes are DNA methylation and histone modification.
“DNA methylation and histone modifications are the most significant epigenetic changes described in gene transcription, linked to the skeletal muscle transcriptional response to exercise, and mediating the exercise adaptations.”
DNA Methylation is when a CH3 methyl group is added or removed from the Cytosine portion of the ATCG configuration of DNA, generally at CpG islands.
- Methylation generally turns Genes OFF
- Demethylation generally turns Genes ON
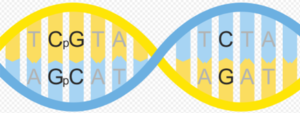
Image: CpG sites are “regions of DNA” within the A-C-G-T scaffolding “where a cytosine nucleotide is followed by a guanine nucleotide.”
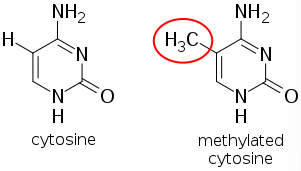
Image: DNA Methylation is when a CH3 methyl group is added or removed from the Cytosine portion of the ATCG configuration of DNA
Histone Modification is when some protein regulators and factors make the histones more loosely or tightly packed, thereby making them either more or less accessible by other proteins that ‘read’ the gene, thereby turning the Gene ON or OFF.
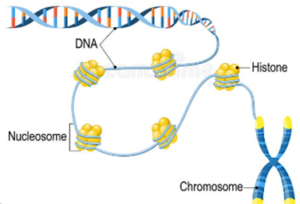
Image: Is the DNA densely packed or loosely packed? Just as storing a garden hose is often easier when using something to coil around it, storing DNA more densely is accomplished by wrapping it around bundles of histones, called nucleosomes. One nucleosome is made up of 8 histone proteins. “The basic repeating structural (and functional) unit of chromatin is the nucleosome.”
More detailed explanations of how exercise triggers genes to turn ON or OFF include:
- Physical exercise triggers mTOR enzymes, which “epigenetically” tells the MTOR gene to produce more mTOR.
- “Many lines of evidence indicate that the hyperactivation of mTOR signaling favors cell and tissue growth” and “mTOR signaling is activated by hormones and growth factors.” “The MTOR gene provides instruction for making the protein called mTOR.”
- “mTOR is one of those things that’s good to have cycled. Sometimes we want to increase it to grow muscle and improve certain aspects of cognition, while the rest of the time [we] want to have low levels to increase longevity, decrease cancer risk, and reduce inflammation.”
- Physical exercise triggers SIRT1 enzymes, which “epigenetically” activates PGC-1α to further upregulate the PPARα gene. “PPAR-alpha regulates the expression of genes involved in fatty acid beta-oxidation and is a major regulator of energy homeostasis.” Activation of this gene also reduces triglyceride levels.
- “SIRT1 activates PGC-1α, to further up-regulate PPARα gene targets.”
- Recall that physical exercise decreases DNMT enzymes, which delays aging and neurodegeneration. Well, diabetic immune cells show an up-regulation (increase) of DNMT1, with cytosine methylation of mTOR regulators (remember that methylation generally reduces activation and transcription), “leading to pathogenic activation of the mTOR pathway and consequent inflammation in diabetic kidneys.”
- Exercise improves IGF2 & H19 expression. IGF2 affects other genes, like SIRT1 (a longevity gene associated with numerous health functions) and PGC-1α (remodels muscle tissue), as well as offsets their downregulation in mothers with gestational diabetes (GDM). Amazingly, both IGF2 and H19 gene location was affected by paternal exercise in a 2022 study on mice in the International Journal of Molecular Sciences, affecting gene expression in the skeletal muscle of the offspring. Authors wrote how remarkable the evaluation of the paternal sperm was, “indicating that exercise-induced epigenetic changes that occurred during germ cell development contributed to transgenerational transmission.” Their conclusion was that exercising dads, prior to conception, should be considered as a strategy to promote metabolic health in the offspring, and that these results would be inherited transgenerationally.
- Exercise improves PGC-1α expression. A 2021 examination in the Journal of Applied Physiology looked at whether the physical exercise of running could negate the adverse effects of an unhealthy high fat diet in both male and female mice parents. Researchers looked at whole genome DNA methylation analyses of PGC-1α. Impaired metabolic homeostasis was negatively affected in control mice, including hypermethylation of Pgc-1α. However, “maternal exercise during gestation mitigates the negative consequences.” The conclusion was that high fat diets would negatively influence the offspring’s metabolic outcomes later in life, but that moms that exercise during pregnancy would reduce the severity of any negative effects.
So, the new molecules now inundate the DNA environment, allowing “epigenetic” changes to turn ON or OFF genes. Epigenetics is defined as the “changes in organisms caused by modification of gene expression rather than alteration of the genetic code itself.” The DNA itself has not been edited in the way that CRISPR can change the ACGT “letters of the book” but the effect can be just as profound: the genes are signaled to produce molecules differently, thereby changing the outcome.
STEP FIVE: The cycle repeats
Molecules alter genes, and genes make more molecules. These Epigenetic mechanisms then further reinforce or repress additional genetic expression (DNA to RNA to PROTEINS).

Physical exercise creates physiological adaptations that are instigated by transcriptional responses (DNA to RNA). “Consequently, changes in key metabolic, regulatory, and myogenic genes in skeletal muscle occur as both an early and late response to exercise, and these epigenetic modifications…trigger those alterations in the transcriptional responses.”
As the behavior continues, so do the subsequent molecules, and subsequent outcomes.
REMINDER:
A 2021 study in the International Journal of Molecular Sciences, reminds us that “full comprehension of the underlying molecular mechanisms involved in the human [body’s] adaptive response to exercise remains to be determined.”
The science of epigenetics is just one of the topics we discuss as a group each month as part of our Holistic Fertility & Pregnancy Safe Program. Learn more about this monthly program for fitness professionals here!
References
2011. Alegria,-Torres, Baccarelli, & Bollati. Epigenetics and lifestyle. Epigenomics. Retrieved 12/12/22 from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3752894/
IMAGE: https://all-free-download.com/free-vector/exercise.html
2020. Chen & Kudryashev. Muscle cells need calcium ions. Max-Planck-Gesellschaft. Structure of RyR1 in native membranes. EMBO Reports. Retrieved 12/12/22 from https://www.mpg.de/14590554/muscle-cells-need-calcium-ions
1975. Szent-Gyorgyi, AG. Calcium regulation of muscle contraction. Biophysical Journal. Retrieved 12/12/22 from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1334730/
2012. Barres, et al. Acute exercise remodels promoter methylation in Human Skeletal Muscle. Cell Metabolism. Retrieved 12/11/22 from https://www.sciencedirect.com/science/article/pii/S1550413112000058
2022. Adenosine Triphosphate, Coenzyme. Britannica. Retrieved 12/12/22 from https://www.britannica.com/science/adenosine-triphosphate
2003. Hardie, Grahame, D. Minireview: The AMP-Activated Protein Kinase Cascade: The Key Sensor of Cellular Energy Status. Endocrinology. Retrieved 12/12/22 from https://academic.oup.com/endo/article/144/12/5179/2880455
2012. Barres, et al. Acute exercise remodels promoter methylation in Human Skeletal Muscle. Cell Metabolism. Retrieved 12/11/22 from https://www.sciencedirect.com/science/article/pii/S1550413112000058
- Pizzino, et al. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Medicine and Cellular Longevity. Retrieved 12/12/22 from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5551541/
- Briones & Touyz. Moderate Exercise Decreases Inflammation and Oxidative Stress in Hypertension. Hypertension. Retrieved 12/12/22 from https://www.ahajournals.org/doi/10.1161/HYPERTENSIONAHA.109.136622
- Kawamura & Muraoka. Exercise-Induced Oxidative Stress and the Effects of Antioxidant Intake from a Physiological Viewpoint. Antioxidants. Retrieved 12/12/22 from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6162669
- Barres, et al. Acute exercise remodels promoter methylation in Human Skeletal Muscle. Cell Metabolism. Retrieved 12/11/22 from https://www.sciencedirect.com/science/article/pii/S1550413112000058
(n.d.) Gene Expression. Scitable by NatureEducation. Retrieved 12/12/22 from https://www.nature.com/scitable/topicpage/gene-expression-14121669/
- Poitout, et al. Regulation of the Insulin Gene by Glucose and Fatty Acids. The Journal of Nutrition. Retrieved 12/13/22 from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1853259
(n.d) Overview: Eukaryotic Gene Regulation. Khan Academy. Retrieved 12/13/22 from https://www.khanacademy.org/science/ap-biology/gene-expression-and-regulation/regulation-of-gene-expression-and-cell-specialization/a/overview-of-eukaryotic-gene-regulation
Image: Genome Research Limited. Retrieved 12/13/22 from https://www.yourgenome.org/facts/what-is-gene-expression/
IMAGE: https://www.pinterest.com/pin/477874210455815431
https://www.genecards.org/cgi-bin/carddisp.pl?gene=PPARGC1A
https://skeletalmusclejournal.biomedcentral.com/articles/10.1186/s13395-020-00231-8
- Chan & Arany. The Many roles of PGC-1α in Muscle – Recent Developments. Metabolism. Retrieved 12/13/22 from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4040247
- Wong, et al. Nuclear receptor corepressor complexes in cancer: mechanism, function and regulation. American Journal of Clinical and Experimental Urology. Retrieved 12/13/22 from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4219314/
Shimizu, et al. Nuclear corepressor SMRT is a strong regulator of body weight independently of its ability to regulate thyroid hormone action. PLOS ONE. Retrieved 12/13/22 from https://journals.plos.org/plosone/article/file?id=10.1371/journal.pone.0220717&type=printable
- Astapova, Inna. Hollenberg, Anthony. The In Vivo Role of Nuclear Receptor Corepressors in Thyroid Hormone Action. Retrieved 2/6/23 from https://doi.org/10.1016/j.bbagen.2012.07.001
- McGee, Sean. Walder, Ken. Exercise and the Skeletal Muscle Epigenome. Cold Spring Harbor Perspectives in Medicine. Retrieved 2/6/23 from www.ncbi.nlm.nih.gov/pmc/articles/PMC5580508/
https://www.sciencedirect.com/topics/medicine-and-dentistry/dna-methyltransferase
- Xu, et al. Roles of physical exercise in neurodegeneration: reversal of epigenetic clock. Translational Neurodegeneration. Retrieved 12/13/22 from https://translationalneurodegeneration.biomedcentral.com/articles/10.1186/s40035-021-00254-1
https://en.wikipedia.org/wiki/AMP-activated_protein_kinase
- Plaza-Diaz, et al. Impact of Physical Activity and Exercise on the Epigenome in Skeletal Muscle and Effects on Systemic Metabolism. Biomedicines. Retrieved 12/13/22 from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8773693/
- Resistance Exercise-Induced Hypertrophy: A Potential Role for Rapamycin-Insensitive mTOR. Exercise and Sport Sciences Reviews. Retrieved 12/13/22 from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6659995
https://www.sciencedirect.com/topics/medicine-and-dentistry/sirtuin
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6600260/
- Wang, et al. Pyruvate dehydrogenase kinases (PDKs): an overview toward clinical applications. Bioscience Reports. Retrieved 12/13/22 from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8026821/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1665109
- Regular, Intense Exercise Training as a Healthy Aging Lifestyle Strategy: Preventing DNA Damage, Telomere Shortening and Adverse DNA Methylation Changes Over a Lifetime. Frontiers in Genetics. Retrieved 12/13/22 from https://www.frontiersin.org/articles/10.3389/fgene.2021.652497/full
IMAGE: https://medicine.wustl.edu/news/ability-turn-off-genes-brain-crucial-learning-memory/
- Plaza-Diaz, et al. Impact of Physical Activity and Exercise on the Epigenome in Skeletal Muscle and Effects on Systemic Metabolism. Biomedicines. Retrieved 12/13/22 from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8773693/
(n.d.) CpG site. Wikipedia. Retrieved 11/16/22 from https://en.wikipedia.org/wiki/CpG_site
(n.d.) CpG site. Wikipedia. Retrieved 11/16/22 from https://en.wikipedia.org/wiki/CpG_site
https://en.wikipedia.org/wiki/DNA_methylation
- Annunziato, Anthony. DNA Packaging: Nucleosomes and Chromatin. Nature Briefing. Retrieved 12/13/22 from https://www.nature.com/scitable/topicpage/dna-packaging-nucleosomes-and-chromatin-310/
- Wand & Proud. The mTOR pathway in the control of protein synthesis. Physiology. Retrieved 12/13/22 from https://journals.physiology.org/doi/full/10.1152/physiol.00024.2006
- MTOR gene. MedlinePlus. Retrieved 12/13/22 from https://medlineplus.gov/genetics/gene/mtor
- Yazdi, Puya, MD. Medically reviewed by the Self Decode Science Team. All About mTOR + Natural mTOR Inhibitors & Activators. Self Hacked. Retrieved 12/13/22 from https://selfhacked.com/blog/mtor-natural-mtor-inhibitors/
- Van Raalte, et al. Peroxisome proliferator-activated receptor (PPAR)-alpha: a pharmacological target with a promising future. Pharmaceutical Research. Retrieved 12/13/22 from https://pubmed.ncbi.nlm.nih.gov/15497675/
- Tyagi, et al. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. Journal of Advanced Pharmaceutical Technology & Research. Retrieved 12/13/22 from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3255347/
- Chang & Guarente. SIRT1 and other sirtuins in Metabolism. Trends in Endocrinology and Metabolism. Retrieved 12/13/22 from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3943707/
- Chen, et al. Aberrant DNA methylation of mTOR pathway genes promotes inflammatory activation of immune cells in diabetic kidney disease. Kidney International. Retrieved 12/13/22 from https://pubmed.ncbi.nlm.nih.gov/31101365/
- Zhu, et al. IGF2 deficiency causes mitochondrial defects in skeletal muscle. Clinical Science (London, England: 1979). Retrieved 12/13/22 from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8055961/
- Kim, et al. Knockout of longevity gene Sirt1 in zebrafish leads to oxidative injury, chronic inflammation, and reduced life span. PLOS ONE. Retrieved 2/7/23 from https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0220581
- Liang, Huiyun. Ward, Walter. PGC-1alpha: a key regulator of energy metabolism. Advances in Physiology Education. Retrieved 2/7/23 from https://pubmed.ncbi.nlm.nih.gov/17108241/
- Mambiya, et al. The Play of Genes and Non-genetic Factors on Type 2 Diabetes. Frontiers in Public Health. Retrieved 2/7/23 from https://www.frontiersin.org/articles/10.3389/fpubh.2019.00349/full
- Costa-Junior, et al. Paternal Exercise Improves the Metabolic Health of Offspring via Epigenetic Modulation of the Germline. International Journal of Molecular Sciences. Retrieved 12/13/22 from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8744992/
- Laker, et al. Exercise during pregnancy mitigates negative effects of parental obesity on metabolic function in adult mouse offspring. Journal of Applied Physiology. Retrieved 12/13/22 from https://journals.physiology.org/doi/full/10.1152/japplphysiol.00641.2020
- Zhang, et al. Mechanism of methylation and acetylation of high GDNF transcription in glioma cells: A review. Heliyon. NIH. Retrieved 12/13/22 from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6595186
- Plaza-Diaz, et al. Impact of Physical Activity and Exercise on the Epigenome in Skeletal Muscle and Effects on Systemic Metabolism. Biomedicines. Retrieved 12/13/22 from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8773693/
- Swiatowy, et al. Physical Activity and DNA Methylation in Humans. International Journal of Molecular Sciences. Retrieved 12/11/22 from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8657566/

